Maize Genetics Cooperation Newsletter vol 86 2012
DNIPROPETROVSK, UKRAINE
Institute
of Agriculture of Steppe Zone of NAAS of Ukraine
Callusogenesis in maize inbred DK212 under sodium
chloride
Derkach, KV, Abraimova OE, Satarova TM
The
resistance to salinity in maize is the important agricultural characteristics.
Sodium chloride is one of the basic components of saline soils. The application of in vitro tissue culture method
for creation of genotypes resistant to chloride salinity is a perspective
direction of biotechnology investigations (Urechean, Bulg.
J. Plant Physiol., Special Issue: 336-352, 2003). Regenerated plants resistant
to sodium chloride
have been obtained in barley (Ignatova,
2011), rice (Priya et al., Afr. J. Biotechnol. 10(36): 6947-6953, 2011), beet (Chugunkova, Physiol. and Biochemistry of Cultivated plants
6(242): 509-515,2009), wheat (Koutoua et al., Int. J.
Biosci. 1(4):12-25, 2011), potato (Homayoun et al., American-Eurasian J. Agric.&Envinon. Sci. 11(5):729-732,
2011).
The
increase of contents of
sodium and calcium ions, the decrease of the concentration of
potassium ions and the significant decrease of ratio К+/Na+ take place in
tissues under salinity. Higher activity of superoxidedismutase was discovered in salt resistant forms and
the decrease of enzyme activity under abiotic stress were revealed both in
seedlings and callus tissues (Terletskaja, Biology of
Plant Cells in Vitro and Biotechnology: 390, 2008). The salinity of the
environment breaks osmotic and ionic homeostasis of cells and provokes the
formation of a secondary oxidative stress. It is connected with the
accumulation of active forms of oxygen whose oxidation effect is directed to
lipids and leads
to the disruption of structure and functions of membranes [Gurr et al., 2002]. Tissue culture method allows to simulate
the salinity stress through adding sodium chloride to nutrient medium. Such a
medium can be considered
a selective one for
screening of resistant maize cells and tissues to obtain resistant regenerants.
We
investigated the callusogenesis under salinity in maize inbred DK212 which
belonged to subplasm Oh43 of Lancaster germplasm. Immature zygotic embryos,
1-1.5 mm in length, were harvested on the 11th day after self-pollination from
field donor plants and cultivated scutellum up on
modified inductive N6 medium (medium Ind) in the darkness. Calli
derived in 60 days in culture were transplanted to next modified MS media:
control medium (C), control medium + 0.1 Mol/l sodium chloride (6C) and control medium + 0.5 Mol/l sodium chloride (30C) and cultivated at the light. Medium
Ind as
compared to media C, 6C and 30C had contained less sucrose for delaying the osmotic load
because the osmotic pressure was created later with sodium chloride.
50.00
and 20.45 percent of green calli were observed respectively on media C and 6C at the 30th day of cultivation at the light. Green coloration
was disappearing through 30 days after its appearance.
Visually
changes in calli sizes depending on sodium chloride contents were noted only to
the 60th day of cultivation at the light. For estimation of callus cultivation
specific diameters and specific raw weights of calli were measured (fig.1, 2). Specific dry weights
and humidity of calli were determined at the end cultivation
(table).
Values of
specific raw weights and diameters of calli after 60 days of cultivation at the
light were appreciably differed from analogical values fixed before
transplantation. Maximal value of specific raw weight of calli was observed on
the 120th day of cultivation at the light in all variants of the experiment.
The specific diameter values achieved to the 150th day were preserved to the
210th day of cultivation at the light. The tendency of the decrease of specific
dry callus weight and humidity under the increase of sodium chloride concentration
was observed. Such a tendency indicates the inhibitory effect of sodium
chloride on the accumulation of dry weight of calli (table). The reduction of calli humidity
related to salt contents in the medium characterizes sodium chloride as an
osmotic agent.
Thus,
inhibitory effect of sodium chloride on callus growth depends on the age of
culture and the sodium chloride concentration. 0.5 M sodium chloride is
non-lethal concentration for tissue culture of maize inbred DK212 and can be
further examined as a selective one.
Table
Maize callus characteristics on the 210th day of in vitro culture
under different concentrations of NaCl
|
Concentration of
NaCl in the medium, g/l |
Number of calli
cultivated |
Specific raw
callus weight, mg |
Humidity of
calli, % |
|
0 |
44 |
7.42 � 0.98 |
95,01 �
6,64 |
|
6 |
44 |
6.07 � 1.06 |
93,96 �
7,26 |
|
30 |
45 |
2.60 � 0.45 |
91,65 �
8,34 |
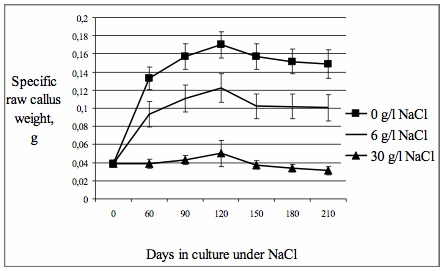
Fig. 1. The dynamics of specific raw
callus weight under the influence of NaCl.
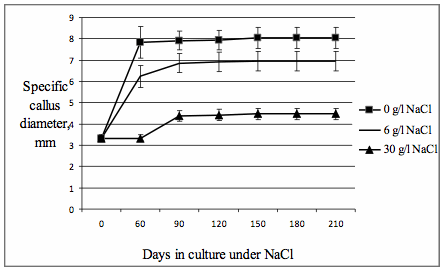
Fig. 2.
The dynamics of specific
callus diameter under the influence of NaCl.
Please Note: Notes submitted to the Maize Genetics
Cooperation Newsletter may be cited only with consent of authors.