Maize Genetics Cooperation Newsletter vol 88
2014
The Maize–Gamagrass Hybrids (2n = 56, 20Zm + 36Td)
Carrying Genomes of the Lines Used for Obtaining Heterosis F1
(
P. A. Panikhin1, E. A.
Abdyrakhmanova1, 3, C. A. Blakey2, and V. A. Sokolov1,
3
1 Cytology and Apomixis Laboratory, Institute of
Molecular and Cellular Biology, pr. Akad. Lavrentieva
8/2, Novosibirsk,
630090 Russia
2 Department of Biology, Ball State
University, Muncie, IN 47306-0440, USA
3 Vavilov Institute of Plant
Industry, ul. Bol�shaya Morskaya 44, St. Petersburg, 190000 Russia
Continuing our study of apomixis in the hybrids between the maize (Zea mays, 2n = 2x = 20)
and gamagrass (Tripsacum
dactyloides, 2n = 4x = 72),
we set the challenge to obtain a new set of 56-chromosome
hybrids (2n = 56 = 20Zm + 36Td).
Note that the main goal of this work was to combine in their genomes two haploid sets of the maize lines used for
obtaining heterosis hybrids. The background for setting
such goal was the earlier studies demonstrating that at least nine chromosomes of the wild parent were necessary for
maintaining an apomictic reproduction in maize–gamagrass hybrids (Sokolov et
al., 1998; Sokolov and Khatypova,
2000). This quantity of the gamagrass genetic material is able to considerably alter the
expression of heterosis; in this case, its fixation
via adopting the elements of apomixis from the wild
relative should be regarded as groundless.
The 573MV and Kubanskaya
611SV lines (analogs of В73 and Mo17, respectively, utilized in breeding
commercial heterosis hybrids) were used as cultivated
parents. To obtain the F1 progeny, the chosen lines were pollinated with the
pollen of tetraploid gamagrass
earlier used to produce apomictic hybrids (Fig. 1).
The seed setting rate in the
crosses where maize lines were pollinated with gamagrass
was rather high, allowing for producing a considerable number of F1 hybrids
(Figs. 2 and 3). However, this rate is
somewhat lower as compared with that observed in the case of gamagrass self-pollination (Table 1). Note that a low
percentage of set seeds observed in the years of 2009 and 2013 was associated with adverse weather conditions, which
prevented an optimal pollination. Then the produced F1 seeds were planted in
fields of the Kuban� experimental station (Institute of Plant Industry); the
grown plants (Figs. 4 and 5) were used in two variants of hybridization, namely, (1)
pollination with the maize line giving heterosis F1 hybrids with the cultivated parent (Table 3) and (2)
backcrossing with the cultivated parent (Table 2). The hybrids obtained in
the first variant (2n = 56 = 20Zm + 36Td)
will be used for assessing the expression of heterosis
and the hybrids of the second variant analogous in their ploidy
will serve as the control. When commencing this study, we expected to obtain segregation
for the genes controlling apomixis, since the
apomictic plants are heterozygous for this
character. Thus, only part of the produced 46-chromosome
hybrids (2n = 10Zm + 36Td) will
further display this type of progeny production. Correspondingly,
only the apomictic F1 plants will massively give functional caryopses after
pollination with the maize. The remaining plants (non-apomictic) will develop
sexual embryo sacs with the chromosome sets
unbalanced as a result of meiosis and will mainly give inviable
seeds.
This particular pattern was
observed in the progenies of F1 hybrids pollinated with maize lines 573 and 611
(Tables 2 and 3).
Two classes of hybrids contrasting
in the seed setting rate are evident in tables, namely, with a rate less than
5% and exceeding 20%.
The intermediate class with a
seed setting rate of about 10% (7.7% in Table 2 and 11.5% in Table 3)
is likely to result from concurrent development
of sexual and diplosporic embryo sacs in a pollinated
line. These data require further verification by hybridization
with tester lines and cytological analysis.
The fact is that an Antennaria-type apomixis,
observed in the gamagrass, is a genetically complex trait. We have earlier demonstrated
that non-reduction (diplospory) and parthenogenesis
in it are controlled in an independent manner (Sokolov,
2000) and the latter is likely to be subject to a polygenic control (Blakey et al., 2007). That is why the used gamagrass pollen may carry different combinations of the
major and minor genes involved in the control of apomixis.
As a result, this gives a fuzzy pattern of the well-filled grains in BC1
generation. Backcrossing of the apomictic F1 plants gives BIII hybrids (2n = 56 = 20Zm + 36Td),
necessary for our further studies, at a rate of approximately
3–5% (Fig. 6). Therefore, their
reliable production requires several hundreds of F1 plants. Currently, the
available amount of seeds is sufficient for producing the necessary number of BIII
hybrids of BC1 generation (Table 1).
References
1.
Sokolov, V.A., Kindigir, B, and Khatypova, I.V.,
Apomictically reproducing 39-chromosome
maize–Tripsacum
hybrids, Genetika, 1998, vol. 34, no.
4, pp. 499–506 [in Russian].
2.
Sokolov, V.A. and Khatypova, I.V., The development of apomictic maize:
update, problems and perspective, Acta
Biol. Yugoslavica, Series F. Genetika,
2000, vol. 32, no. 3. pp. 331–353.
3.
Sokolov, V.A..
Independent inheritance and expression of apomeiosis and
parthenogenesis in maize–gamagrass hybrids, Dokl.
Ross. Akad. Nauk, 2000,
vol. 374, no. 2, pp. 280–282 [in
Russian].
4.
Blakey, C.A., Costich, D., and Sokolov V.A., Tripsacum research: a perspective from observation
along a river to molecular genomics, Maydica,
2007, vol. 52, pp. 81–99.
FIGURE
SUMMARY
Figure 1. Ears of maize
lines, their F1 reciprocal hybrids, and 46-chromosome
F1 maize–gamagrass
hybrids (line 611 × T. dactyloides
and line 573 × T. dactyloides).
IN FIGURE:
F1 Kubanskaya
611SV × T. dactyloides, 46 chromosomes (10Zm + 36Td)
self pollinated line Kubanskaya
611SV
F1 Kubanskaya
611SV × 573MV
F1 573MV × Kubanskaya 611SV
self pollinated line 573MV
F1 573MV × T. dactyloides, 46 chromosomes
(10Zm + 36Td)
Figure 2. Seeds of 46-chromosome
F1 maize–gamagrass
hybrids (line 611 × T. dactyloides
and line 573 × T. dactyloides).
Figure 3. Seeds of line 611 and 46-chromosome F1 maize–gamagrass hybrid (line 611 × T. dactyloides).
Figure 4. Inflorescences of T. dactyloides (left) and 46-chromosome
F1 maize–gamagrass
hybrid (right).
IN FIGURE:
Inflorescence of T. dactyloides (2n = 4x = 72)
Ear of F1 hybrid line
573 × T. dactyloides, 46 chromosomes (10Zm + 36Td)
Figure 5. A plant of 46-chromosome
F1 maize–gamagrass hybrid (line 573 × T. dactyloides).
IN FIGURE:
46-chromosome F1 maize–gamagrass
hybrid line 573 × T. dactyloides,
plant no. 4.8
Figure 6. A plant of 56-chromosome
maize–gamagrass hybrid with a genomic
composition of 10Zm (line 573) + 10Zm (line 611) + 36Td.
IN FIGURE:
BC1 of 46-chromosome F1
maize–gamagrass hybrid (line 573 × T. dactyloides) × line 611, plant
no. 37.2
TABLES.
Table 1.
Seed setting rates
of the maize lines 573 and 611 pollinated with T. dactyloides
and self-pollinated gamagrass plants (2n = 4x = 72)
|
Pollination variants |
Year |
Number of pollinated
ears |
Number of set ears |
Number of unset ears |
Number of flowers |
Number of grains |
Seed setting rate, % |
|
573 х T. dactyloides |
2010 |
5 |
5 |
0 |
3040 |
890 |
29.3 |
|
611 х T. dactyloides |
1 |
1 |
0 |
580 |
237 |
40.9 |
|
|
573 х T. dactyloides |
2011 |
11 |
11 |
0 |
6688 |
2031 |
30.4 |
|
611 х T. dactyloides |
17 |
17 |
0 |
9945 |
2015 |
20.3 |
|
|
573 х T. dactyloides |
2012 |
70 |
70 |
0 |
42589 |
10810 |
25.4 |
|
611 х T. dactyloides |
18 |
18 |
0 |
10442 |
5596 |
53.6 |
|
|
T. dactyloides self-pollination |
2009 |
93 |
28 |
65 |
852 |
78 |
9.2 |
|
T. dactyloides self-pollination |
2010 |
186 |
173 |
13 |
2076 |
1422 |
68.5 |
|
T. dactyloides self-pollination |
2012 |
154 |
154 |
0 |
1719 |
1031 |
60.0 |
|
T. dactyloides self-pollination |
2013 |
65 |
42 |
23 |
763 |
109 |
14.3 |
Table 2.
Seed setting rates
of the 46-chromosome F1 maize–gamagrass hybrids
line 573 (Zea mays) × T. dactyloides backcrossed with line 573
|
BC1 variants |
Year |
Number of pollinated ears |
Number of flowers |
Number of set grains |
Number of filled grains |
Seed setting rate, % |
|
F1 no. 1.9 х 573 |
2012 |
2 |
16 |
7 |
6 |
43.8 |
|
F1 no. 3.8 х 573 |
3 |
34 |
17 |
13 |
50 |
|
|
F1 no. 3.15 х 573 |
3 |
26 |
2 |
1 |
7.7 |
|
|
F1 no. 4.8 х 573 |
6 |
88 |
1 |
0 |
1.1 |
|
|
F1 no. 3.8 х 573 |
2013 |
15 |
193 |
39 |
29 |
20.2 |
|
F1 no. 3.9 х 573 |
2 |
25 |
0 |
0 |
0 |
|
|
F1 no. 3.15 х 573 |
2 |
29 |
0 |
0 |
0 |
Table 3.
Seed setting rates
of the 46-chromosome F1 maize–gamagrass hybrids
line 573 (Zea mays) × T. dactyloides backcrossed with line 611
|
BC1 variants |
Year |
Number of pollinated ears |
Number of flowers |
Number of set grains |
Number of filled grains |
Seed setting rate, % |
|
F1 no. 1.9 х 611 |
2012 |
8 |
81 |
31 |
24 |
38.3 |
|
F1 no. 3.8 х 611 |
12 |
159 |
78 |
58 |
49.1 |
|
|
F1 no. 3.9 х 611 |
1 |
25 |
1 |
0 |
4 |
|
|
F1 no. 3.15 х 611 |
5 |
76 |
1 |
0 |
1.3 |
|
|
F1 no. 4.8 х 611 |
12 |
192 |
0 |
0 |
0 |
|
|
F1 no. 1.9 х 611 |
2013 |
24 |
275 |
105 |
83 |
38.2 |
|
F1 no. 3.8 х 611 |
44 |
603 |
212 |
161 |
35.2 |
|
|
F1 no. 3.9 х 611 |
11 |
139 |
16 |
9 |
11.5 |
|
|
F1 no. 3.15 х 611 |
10 |
115 |
3 |
3 |
2.6 |
|
|
F1 no. 4.8 х 611 |
58 |
672 |
3 |
2 |
0.5 |
Figure 1. Ears of maize
lines, their F1 reciprocal hybrids, and 46-chromosome
F1 maize–gamagrass
hybrids (line 611 × T. dactyloides and line 573 × T. dactyloides).
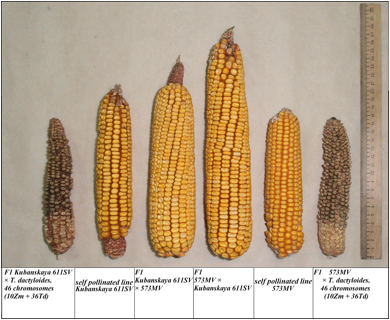
Figure 2. Seeds of 46-chromosome F1 maize–gamagrass hybrids (line 611 × T. dactyloides and line 573 × T. dactyloides).

Figure 3. Seeds of line 611 and 46-chromosome F1 maize–gamagrass hybrid (line 611 × T. dactyloides).

Figure 4. Inflorescences of T. dactyloides (left) and 46-chromosome F1 maize–gamagrass hybrid (right).


Figure 5. A plant of 46-chromosome
F1 maize–gamagrass hybrid (line 573 × T. dactyloides).

Figure 6. A plant of 56-chromosome maize–gamagrass hybrid with a genomic composition of 10Zm (line 573) + 10Zm (line
611) + 36Td.

Please Note: Notes
submitted to the Maize Genetics Cooperation Newsletter may be cited only with
consent of authors.