Maize
Genetics Cooperation Newsletter vol 87 2013
Maize mutants a boon to society in past
and in future what�?
Mahak Tufchi, N. K.
Singh*, A. Yadav, G. Tiwari, S.S. Verma, J.P. Jaiswal, P. K. Shrotria, A. K.
Gaur, Anil Kumar
G. B. Pant University
of Agriculture & Technology, Pantnagar-263145 (Udhamsingh Nagar)
Uttarakhand, India
*email:
[email protected]
Abstract
Any
altered plant phenotypic expression is a variation that may be desirable or
undesirable. Mutants form one such class which has been exploited in past and
have role to play in future. One of the aspects in utilizing the worth of
mutants is conversion of normal maize into quality protein maize (QPM). In the current
investigation we have found a number of plants in the BC2F1
populations whose phenotypic expression were different from normal plants. The
most susceptible population was BC2F1/11 where frequencies
of altered phenotypes were maximum (42.42 %) whereas minimum of 2.08 % plants
were noted with altered phenotypes in BC2F1/2. Further,
altered plant phenotypes were grouped according to their similarity to identify
the severe type phenotypic alteration. Based on grouping, cob at top without
tassel was identified to be the most frequent one with 25 plants showing such
types of phenotypic alteration. The unusual phenotypes found minimum was one
each in earless, tasseless with cob cluster, branched and cob and tassel
together at top mutants.
Introduction
Maize
is a model biological system as well as an important agronomic crop. While it
is anticipated that novel mutants that enhance our understanding of maize as a
biological organism will lead to applications that improve this plant as a
crop, it is also clear that some information from basic maize mutant research
can have direct applications. One classic example is the discovery that mature shrunken2
(sh2) mutant kernels are sweet (Laughnan, 1953). This led to a
revolutionary change in the sweet corn industry, the transition from the
traditional sugary varieties to the sh2-based super-sweet varieties
(Tracy, 1997). This turned fresh sweet corn from a local seasonal crop to one
that can be enjoyed year round. Laughnan (1953) originally used the sh2 mutant
because of its tight genetic linkage to the anthocyanin pigment synthesis
factor, a1, an early example of marker-assisted selection. More
recently, it was discovered that sh2 encodes the 60 kDa subunit of
endosperm ADP glucose pyrophosphorylase, an enzyme essential for starch
synthesis (Bhave et al., 1990). Other mutants such as sugary1,
sugary enhancer1, opaque2, floury2, waxy1, amylose extender1, Leafy1,
and brown midrib3, have also been used in direct applications for
specialty corn production (Cox and Cherney, 2001; Hallauer, 2001).
In
maize, the shoot apical
meristem (SAM) typically initiates a fixed number of leaves during the
vegetative phase of growth. The initiation of many leaves is accompanied by the
formation of a new meristem in its axil called the axillary meristem. The shift
from vegetative to reproductive development is a significant transition in the
maize life cycle. During this transition, the SAM ceases to make leaves and
transforms into an inflorescence meristem, which subsequently produces a number
of specialized lateral meristems that ultimately lead to the formation of the
tassel (male inflorescence) (Russell and Stuber, 1983; Irish and Nelson, 1991).
The tassel is a branched inflorescence consisting of one central spike and
several basal branches that together bear the spikelets and florets containing
the floral organs. To initiate tassel formation, the inflorescence meristem
gives rise to several files of branch meristems spanning its entire length.
These branch meristems either elongate to form long lateral branches or become
spikelet pair primordia, which branch again to form two spikelet meristems.
Each spikelet meristem goes on to initiate a pair of floral meristems that give
rise to the floral organs (Kiesselbach, 1949; Cheng et al., 1983). In contrast to the tassel, ears (female
inflorescences) develop from one or more axillary meristems on the main axis of
the plant. The ear develops as a single thick rachis without basal branches
(Kiesselbach, 1949; Veit et al.,
1993; McSteen et al., 2000). There
exists in maize several curious mutants where one or the other inflorescence
fails to form altogether. The tasselless1 (tl1) mutants lack a
tassel at maturity, but can produce ears and may otherwise appear normal.
Anecdotal evidence suggests that this is a phototropic response that can be
ameliorated by growth under short days, but detailed studies on this mutant are
lacking. Similarly, the mutants barren stalk2 and 3 lack ears and
tillers but produce an otherwise normal plant including a normal tassel (Pan
and Peterson, 1992; Neuffer et al.,
1997). In the case of ba2, at least, ears shoots are initiated but then
arrest their development at such an early stage that the shoot never emerges
from the axil of the leaf. Unlike ba1, which affects initiation of all
axillary meristems, ba2 and ba3 are specific to the axillary
shoot meristem, but affect the continued growth of the shoot rather than its
initiation or formation. The recessive andromonoecious dwarfs fail
to synthesize normal levels of gibberellic acid (GA) and normal stature and
floret development can be rescued by GA application (Phinney, 1956; Phinney and
West, 1960). The group of tasselseed mutants shares in
common the partial to nearly complete conversion of the normally male tassel
spikelets into ones that bear female florets (Nickerson and Dale, 1955). This
often results in seeds being formed on the tassel, sometimes as profusely as on
the ear. Two groups of tasselseeds were established based on genetic analyses
and morphological considerations
with ts1, ts2, Ts3, and Ts5 falling in the
group where sex reversal was not accompanied by extra branching, and ts4 and
Ts6 affecting sex determination and causing proliferative inflorescence
branching (Irish et al., 1994). The ts2
was the first tasselseed gene to be cloned (DeLong et al., 1993). It encodes a short chain alcohol dehydrogenase that
is expressed in both ear and tassel spikelets. This leads to an apoptotic-based
degeneration of the gynoecium in the tassel florets as well as in the lower
floret of the ear (Calderon-Urrea and Dellaporta, 1999).The silky1 (si1)
mutant which produces numerous silks emerging from both tassel and ear
spikelets. A mutant tassel producing silks is reminiscent of the tasselseed
class of mutants. However, in the case of si1 mutants the silks arise in
place of stamens due to homeotic floral organ conversions. The si1 encodes
a MADS-box gene related to the B-class floral homeotic MADS-box genes APETALA3
of Arabidopsis and DEFICIENS of Antirrhinum (Ambrose et al., 2000).
The
discoveries that arise from research on maize mutants will undoubtedly enhance
our understanding of maize as a biological organism. Investigation on diverse
allelic variants will probably lead to other revolutionary enhancements that
can be applied to maize and other crop plants to help feed humanity in the
future. Considering the significance of allelic variants in maize improvement,
we characterised the various types of altered plant phenotypes appeared in BC2F1
population, developed for conversion of normal maize into quality protein maize
(QPM).
Materials and methods
The
materials comprised of BC2F1 populations derived from
crosses between Pant 10k1375 x CML161 and backcrossed with recurrent parent
(Pant 10k1375). The Pant10K1375, a normal maize inbred line, developed in Maize
Breeding Programme at Pantnagar. The CIMMYT Maize Line 161 (CML-161), a QPM
inbred lined, was developed by CIMMYT. The F1 of initial cross were backcrossed
with recurrent parent, and BC1F1 and BC2F1
plants were selected for heterozygous opaque2
allele (o2) using SSR markers for conversion of normal maize line (Pant
10k1375) into QPM. Progeny populations of all the 12 plants found positive
based on the phi057 and umc1066 SSR
markers for o2 allele were planted on
09.09.2012. The selected plants were self pollinated to generate the BC2F2
populations for conversion programme. Out of the progenies population of 12
plants, initially selected for heterozygous o2
locus, some were found to have altered phenotypic expression than the normal
type. The phenotypic differences were related to plant height, position of ear
placement, differential expression of reproductive organs. Such kind of changes
were across the progeny populations of 12 plants characterized for plant
height, number of ears, effective ears, barren ears, branching pattern.
Results and discussion
A
global climatic change is now considered to be underway and is expected to
result in a long term trend towards changes in environmental conditions.
Congenial environmental seasons support optimal development, however, unfavourable
environments influence the genetic architecture of the plant and reduce yield
directly by affecting plant growth and development, and indirectly by modifying
the normal plant phenotype. On the other hand, altered plant phenotype may
serve as valuable source of variability for improvement of specific trait or
even can help in development of specific purpose corn as happened in past in
development of specialty corn such as sweet corn, QPM etc. Unpredictability of
weather conditions has occasionally resulted in many unusual expressions in
plant characteristics in general, and ear and tassel characteristics in
particular, in maize. Multiple ears on single nodes are one of the
environmentally induced oddities widely reported in maize. The expression of multiple ears in inbred
lines, populations and experimental hybrids was also recorded in maize grown in
the Tarai region of Uttarakhand, India (Singh et al., 2009; Singh and Devi, 2010). The twin ear expression on 33
single nodes in maize was observed earlier by Hallauer in 1973 in S2 and S5
progenies of two populations (Hallauer, 1984). The unusual expressions were observed
in BC2F1 population planted during September and flowered
during early November 2012. The temperature during the sowing and initial
vegetative growth was normal whereas during pre-flowering, flowering,
post-flowering and grain filling stages, the temperature was lower than the
normal required for maize. The unusual expressions include the expression of
silks in tassel, part of the tassel converted into an ear, plants with terminal
ears without any tassels, earless and terminal as well basal branching. In
fact, unisexuality in maize occurs through the selective elimination of stamens
in ear florets and by elimination of pistils in tassel florets. The two general
classes of sex determining mutants have been identified in maize, including
those of masculinized ears and feminized tassels. The endogenous gibberellic
acid (GA) has been found to have a feminizing role in sex determination in
maize (Tanurdzic and Banks, 2004). Moreover, reversal of sexual expression in
maize has been shown to be influenced by environment and heredity (Richey and
Sprague, 1932; Heslop-Harrison, 2008). The altered phenotypic expressions
observed in the present investigation have been described on ensuing pages.
The
observations indicate that progenies of BC2F1/4, BC2F1/5,
BC2F1/6 did exhibit plants with altered plant phenotypes.
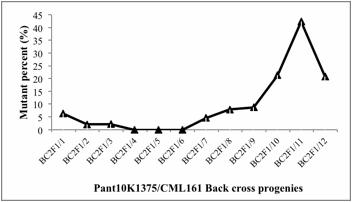
The most susceptible population was BC2F1/11 where
percentage of mutant phenotypes was maximum (42.42 %) whereas minimum
percentage of 2.08 % was found of BC2F1/2 altered phenotypes
(Fig.1). Further, altered plant phenotypes were grouped according to their similarity,
to identify the severe type phenotypic alteration. Based on grouping, cob at
top without tassel was identified to be the most frequent one with 25 plants
showing such types of phenotypic alteration. The unusual phenotypes found
minimum was one each in earless, tasseless with cob cluster, branched and cob
and tassel together at top mutants (Fig.2).
Fig.1: Frequency of mutants in Backcross
progenies in maize
Dwarf mutant
The
heights of these mutants were in the range of 29-77 cm and had presence of
tassel and ear. The ears having normal silk but the tassel was small in the
form of cluster (abnormal). Seed development was observed in these cobs since
other plants having normal tassel development were present in the vicinity of
the plant. Intermodal distance between the nodes is minimized with less leaves.
This may be due to suboptimal synthesis of endogenous gibberellic acid
(Fig.3a).
3b 3b 3a


Fig.3a Dwarf mutant
Fig.3b
Cob and tassel together at top
Tassel and cob
together at top
Tassels with
both anthers and ears. Some plants were found
to have both sexual expressions in the tassel. Generally, the main rachis of
the tassel was converted into a small ear that set seed, whereas the remaining
tassel branches developed anthers with pollen grains along with few seeds on
tassel spikelets (Fig.3b). This type of mutant showed similarity with
tasselseed mutants (Nickerson and Dale 1955).
Cob at top without
tassel
Maize plants normally consist of terminal
tassels as male inflorescences and lateral ears as female inflorescences. In 25
plants, however, the terminal tassels were entirely modified into small single
ear with silk in place of tassels (Fig.3c). These ears were found to have seed
set. However, the size of the cob and number of grain varied from plant to
plant and also smaller than the size and number on normal ear.
3d 3c 3c


Fig.3d Earless
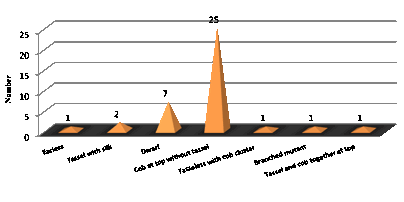
Fig. 2: Frequency of Mutant Types
Earless mutant (Barren stalk 1):
Inflorescence mutant of maize named barren
stalk1 (ba1). barren stalk1 is a recessive mutant of maize that has no
tassel branches, spikelets, tillers, or ears, first identified in 1928
(Hofmeyer, 1930). In the original report, the ba1 mutant was described
as having defective tassel branching and a central stalk devoid of any ears.
ba1 mutants are disrupted in the initiation of both vegetative and
inflorescence axillary meristems. This defect manifests itself as a failure to
produce vegetative branches (tillers), branches in the tassel, spikelets on the
tassel�s central spike, and ears. In the present investigation, we found one
plant having no ear but normal tassel in BC2F1-1 plant
population (Fig.3d).
Branched mutant
Occurrence of lateral and basal branching
was observed in this type of mutant, terminating with a cob either singly or in
clusters. Height of these plants was lower than the normal and completely
lacked the male reproductive part tassel (Fig. 3e).
3g 3f 3e



Fig.3e Branched Fig.3f Tassel with silk
Fig.3g Tasseless with cob cluster
Tassel with silk
The
tassel is the terminal male organ, consisting of anthers and producing pollen
grains for fertilization of the ovule, which is borne in the so-called lateral
ear. In two such plants, rudimentary tassel with profuse silk without ear was
observed. Such plants were also of short height with less intermodal distance
(Fig. 3f).
Tasseless with cob in cluster
A
single plant was found to have many ears on separate nodes without tassel
formation. In such cases, ears were lacking silks and with extended leafy
sheath. As a result, pollination could not take place and ears remained barren
or set very few seeds. Such plants were also branched laterally and also less
in height than the normal plants (Fig. 3g).
The
unusual expressions in ear, tassel, plant height and branching may be due to
environmental factors as the pre-flowering, flowering, post-flowering and grain
filling stages are coincide with the low temperature along with short day
length. There is also probability of involvement of genetic factors or
interaction of genetic and environmental factors. However, progeny analysis or
other genetical studies are required to be carried out to confirm the basis of
plant phneotypes alteration. Such analysis is also essential to assess the
worth of such phenotypically modified plants in maize improvement programme.
Richey and Sprague (1932) reported the role of environment, i.e., shorter daylight periods and lower
temperatures, and heredity in the development of silks in the tassels.
Heslop-Harrison (2008) also shared the viewpoint that low temperatures,
particularly when experienced through the dark period of the daily photoperiodic
cycle, promote female sexual expression and depress male. In case of widespread occurrence of
unusual plant phenotypes, the quality as well as the quantity of the maize
grain or green cob will certainly suffer.
Acknowledgement
Director Experiment Station, G. B. P. U. A
&T. Pantnagar is duly acknowledged for providing facilities to carry out
the experiment.
References
Ambrose
B. A., Lerner D. R., Ciceri
P., Padilla C. M., Yanofsky, M. F., and Schmidt R. J. (2000). Molecular and
genetic analyses of the silky1 gene reveal conservation in floral organ
specification between eudicots and monocots. Molecular Cell 5, 569 – 579.
Bhave
M.R., Lawrence S., Barton C., Hannan C. 1990. Identification and molecular
characterization of shrunken-2 cDNA clones of maize. Plant Cell 2: 581-588.
Calderon-Urrea A., and Dellaporta S. L. (1999).
Cell death and cell protection genes determine the fate of pistils in maize. Development
126, 435 – 441 .
Cheng
P. C., Greyson R. I., and Walden D. B. 1983. Organ initiation and the
development of unisexual flowers in the tassel and ear of Zea mays.
American Journal of Botany 70:
450–462.
Cox
W.J., Cherney D.J.R. 2001. Influence of brown midrib, leafy, and transgenic
hybrids on corn forage production. Agron.
J. 93: 790-796.
DeLong A., Calderon-Urrea A., and Dellaporta S.L.
(1993). Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol
dehydrogenase required for stage-specific floral organ abortion. Cell 74, 757 – 768.
Hallauer
A.R., 2001. Specialty corns. 2nd ed. CRC Press, Boca Raton, FL, 479 pp.
Hallauer A. R. 1984. Twin- ear expression, Maize
Genet. Newsl., 58:21-22.
Heslop-Harrison, J. 1957. The
experimental modification of sex expression in flowering plants. Biology
Reviews, 32: 38–90.
Hofmeyer, J. D. J. 1930. The inheritance and linkage relationships of barren
stalk1 and barrenstalk2, two mature plant characters of maize. Ph.D. Dissertation, Cornell University, Ithaca, New York,
USA.
Irish E. E., Langdale J. A., Nelson T. M. (1994).
Interactions between tassel seed genes and other sex determining genes in
maize. Developmental Genetics 15,
155 – 171.
Irish
E. E., and Nelson T. M. 1991.
Identification of multiple stages in the conversion of maize meristems from
vegetative to floral development. Development 112: 891–898.
Kiesselbach,
T. A. 1949. The structure and reproduction of corn. Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, New York, USA.
Laughnan J. R., 1953. The effect of the sh2 factor
on carbohydrate reserves in the mature endosperm of maize. Genetics 38(5): 485-499.
Mcsteen,
P., Laudencia-Chingcuanco D., and Colasanti J. 2000. A floret by any other
name: control of meristem identity in maize. Trends in Plant Science 5: 61–66.
Neuffer
M. G., Coe E., and Wessler S. R. 1997. The mutants of maize. Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, New York, USA.
Nickerson N. H., and Dale E. E. (1955). Tassel
modifications in Zea mays. Annals of the Missouri Botanical Garden 42, 195 – 211.
Pan
Y. B., and Peterson P. A. 1992. ba3: a new barrenstalk mutant in Zea mays. Journal of Genetics and
Breeding 46: 291–294.
Phinney
B. O. (1956). Growth response of single-gene dwarf mutants in maize to
gibberellic acid . Proceedings of the National Academy of Science, USA 42, 185 – 189.
Phinney
B. O. and West C. A. (1960). Gibberellins and the growth of flowering plants.
In: Developing Cell Systems and Their Control, ed. by D. Rudnick (New
York: Ronald Press), pp. 71 – 92.
Richey,
F. D. and Sprague G. F. 1932. Some factor affecting the reversal of sex
expression in the tassels of maize. Amer.
Nat. LXVI:433-443.
Russell
W. K., and Stuber C. W. 1983. Effects of photoperiod and temperatures on the
duration of vegetative growth in maize. Crop Sciences 23: 847–850.
Singh, N. K. and Devi, P.
2010. Studies on multiple ear trait expression in maize (Zea mays L.). In: Proceedings of the
10th Asian Regional Maize Workshop on Maize for Asia: Emerging
Trends and Technologies (Edited by PH Zaidi, Mohammad
Azarai and K. Pixley).
Makassar, Indonesia Oct 20-23, 2008, Mexico D.F. CIMMYT. P. 130-134.
Singh, N. K., Devi, P. and Mishra, P. 2009. Expression
of unusual characters in ear shoot and tassel of maize. MNL, 83:32-34.
Tanurdzic M, Banks J. A. 2004.
Sex-determining mechanisms in land plants. The Plant Cell
16, S61–S71. 32(1):38-90
Tracy
W.F. 1997. History, genetics, and breeding of supersweet (shrunken2)
sweet corn. Plant Breed. Rev. 14: 189-236.
Veit
B., Schmidt R. J., Hake S., Yanofsky M. F. 1993. Maize floral development: new
genes and old mutants. Plant Cell 5:
1205–1215.
Please Note: Notes submitted to the Maize Genetics
Cooperation Newsletter may be cited only with consent of authors.