Maize Genetics Cooperation Newsletter vol 85
2011
Incidence of male fertility in haploid elite dent
maize germplasm
Geiger HH, Sch�nleben M
University of Hohenheim, Stuttgart, GERMANY
In
vivo haploid induction
presently is a widely used tool in maize research and breeding (Geiger 2009,
pp. 641-657 in Handbook of Maize:
Genetics and Genomics, Springer). However, virtually all haploid plants are
male sterile and therefore cannot be selfed. In
contrast many haploids display a certain degree of female fertility if
pollinated by diploids (Chalyk 1994, Euphytica 79:13-18; Geiger et al 2006, MNL 80:28-29). To fully restore male and female
fertility, the chromosome set of haploids needs to be doubled by mitosis
inhibiting substances like colchicine. But artificial
chromosome doubling is laborious and colchicine is
extremely toxic. Spontaneous chromosome doubling occurs only at a rate of 1-10%
(Chase 1949, Proc Iowa Acad Sci
56:113-115; Beckert, 1994, pp.201-213 in Biotechnology in Agriculture and
Forestry, Springer). In the present newsletter we are reporting about two
experiments in which we determined the male fertility of haploids in a
genetically broad collection of actual elite maize breeding materials. One
experiment was conducted in the field (Exp. I) and one
in the greenhouse (Exp. II).
Experiment I: Materials were comprised of
23 haploid (H) lines which had been development from 22 dent doubled haploid
(DH) lines and 1 flint DH-line by in vivo
haploid induction. The H-lines were as uniform as their parental DH lines but
smaller and less vigorous. All H-lines had been preselected for female
fertility (Geiger et al. 2006, l.c.).
The experiment was laid out in a randomized block design with 2 replicates on
single-row plots of 2.5m length at Stuttgart-Hohenheim in 2007 (Fig.2). Up to 20 seeds were drilled per row. Ten
lines could not be grown in the 2nd replicate due to lack of seed.
No particular stress occurred during the growing season. Male fertility was
assessed on a 1-4 scale defined as follows:
Score 1: One or very few extruding anthers, unbranched tassel.
Score 2: Several extruding anthers at the
central spike; few lateral branches but
without
extruding anthers (Fig.3).
Score 3: Multiple
extruding anthers at the central spike and at most branches but
no
pollen release visible when touching the anthers.
Score 4: Many pollen-shedding anthers at all
branches.
To avoid uncontrolled pollination, all ears were bagged before anthesis. Selfing was tried
whenever a plant displayed any degree of male fertility. For this purpose, we
bagged the tassel in the morning and pollinated the respective plant about four
hours later. Before removing the bag from the tassel, the bagged tassel was
carefully shaken to excite pollen release. No attempts were made to squeeze out
pollen grains from closed anthers.
Experiment II:
Materials consisted of H-plants developed by in vivo haploid induction from each of 70 single, three-way,
double, or multiple crosses between inbred lines of the dent gene pools Iowa
Stiff Stalk, Iodent, Lancaster, Longdent,
and Danube. The crosses represented actual elite dent breeding material. Six
H-plants per cross (420 in total) were raised in a greenhouse at Hohenheim in
10-liter pots during the summer in 2007. The experiment was laid out as a
lattice design with six replicates. Forty-nine plants were removed before anthesis because they looked like heterozygous diploids.
Optimal maize growing conditions (temperature, water supply, light, fertilizer)
prevailed throughout the season. This was reflected in a faster growth and a taller
and more vigorous stature than in the field. Male fertility assessment and selfing were conducted as in Exp. I.
To confirm haploidy, one plant per H-line in
Exp. 1 and all plants in Exp. 2 were analyzed by flow cytometry
(PARTEC instrument CA-II) For this purpose, tissue was taken from the youngest
leaf in the 4-leaf stage. A second analysis was conducted during anthesis of all greenhouse plants with anther score 4.
Tissue was taken from the top leaf. Microsatellite markers phi011, phi072,
umc1153, umc1887, phi032, and phi041, located on chromosomes 1, 4, 5, 6, 9, and
10, respectively (http://maize gdb.org), were used
to check the DH offspring of each successfully selfed
H-plant for genetic uniformity. DNA extraction and marker assessment were
performed according to standard protocols. An ALFexpress
sequencer was used for visualizing the marker genotypes.
Results: In the field, 412 plants displaying the typical morphology of haploids
were inspected for extruding anthers. About half of them (212 plants) conformed
to anther scores 1,2, or 3. They were found in 10 of the 23 tested H-lines. We
attempted to self these plants repeatedly over two or three successive days. Twenty-nine
of them (7.0% of all 412 plants) belonging to 9 lines set one or more seeds per
ear (Table 1). By
far the highest seed set occurred in line 20, in which 10 out of 19 plants could
successfully be selfed. Seed set ranged among these
plants from 1 to 12 (85 seeds in total). In the other 8 lines only 1 to 2
plants per line set seed. A moderate correlation existed between anther score
and seed set (r = 0.56, P < 0.01).
In the greenhouse, 248 out of 371 inspected haploid plants displayed some
degree of male fertility. In all these cases, selfing
was attempted in the same way as in the field experiment. Twenty-seven plants
(7.3% of all tested plants) showed seed set. With three of these plants, the
number of kernels per ear amounted to 23, 16, and 11, respectively, and 9
plants had 5 to 9 seeds per ear (Table 2, Figs.1 and 4). The successfully selfed
plants originate from 21 parental crosses comprising all tested gene pool
combinations. The correlation between anther score and seed set was only weak
(r = 0.30 P
< 0.01).
All visually classified haploids were confirmed by flow cytometry, and in Exp.II
molecular marker analysis attested the genetic uniformity of all progenies
resulting from self-pollination.
Discussion: Results confirm the pioneering work of Chase (1952, pp. in Heterosis.
Iowa State College Press) and later reports of Zabirova
et al. (1993, MNL 67:67), Chalyk (1994 l.c.) and others. Beyond that, our flow cytometry
and molecular marker studies for the first time proved that the selfed plants actually were haploid and gave rise to
genetically uniform progenies. It remains unclear whether the functional male
and female gametes arose by meiotic restitution (Ramana
and Jacobsen 2003, Euphytica 133, 3-18) or by
chromosome doubling due to endomitosis in meristematic cells of the reproductive tissue leading to
diploid sectors in the gametophyte (Chase 1952, l.c.).
In both of our experiments, we found significant genetic variation for
male fertility and selfing success in haploids.
Recurrent selection (RS) for these characteristics therefore seems promising.
This is supported by results of Zabirova et al. (1993, l.c.) who obtained a remarkable increase of male fertility after
four generations of selection for male fertility in a Russian dent population. Thus
breeders might not need to go back to accessions from the world collection to
improve their elite gene pools in this regard.
In conclusion, our studies indicate that in the long run breeders might
be able to improve the degree of male fertility in haploids to an extent that
would allow them to eventually abolish the laborious and hazardous artificial chromosome doubling step of the present maize DH technology.
Acknowledgements:
The authors are indebted to the seed companies KWS SEED AG and LIMAGRAIN
EURROPE for providing the haploid seeds used in Exps.I
and II, respectively. Furthermore, we acknowledge the careful technical
assistance of J. Jesse (field), H. Brandt and S. Spiess
(greenhouse), M. Takacs (marker lab), and M.D. Braun
(flow cytometry).
Table 1. Number of successfully selfed plants
and means across these plants for anther score and number of kernels per ear in
nine haploid dent lines; Exp.I, Hohenheim
2007
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
a For score definitions see text.
b Missing value.
Table 2. Anther score, number of kernels per ear, and plant height of
successfully selfed haploid plants yielding at least five
kernels per ear; Exp.II, Hohenheim
2007
|
Plant |
|
Origina |
Anther |
No. of |
Plant |
|
no. |
|
|
score |
kernels |
height |
|
|
|
|
(1 - 4)b |
per ear |
(cm) |
|
1 |
|
Da/La |
3 |
23 |
162 |
|
2 |
|
Io/La |
3 |
16 |
172 |
|
3 |
|
Ld/SS |
3 |
11 |
160 |
|
4 |
|
Ld/SS |
4 |
9 |
133 |
|
5 |
|
Ld/Io |
4 |
8 |
173 |
|
6 |
|
Ld/Io |
2 |
6 |
113 |
|
7 |
|
NN/SS |
1 |
6 |
138 |
|
8 |
|
La |
2 |
6 |
183 |
|
9 |
|
SS/Ld |
2 |
6 |
125 |
|
10 |
|
Io/La |
1 |
5 |
123 |
|
11 |
|
Io/La |
3 |
5 |
151 |
|
12 |
|
Io/La |
1 |
5 |
180 |
a Da = Danube, Io = Iodent, La = Lancaster, Ld = Longdent,
SS = Iowa Stiff Stalk,
NN = Unknown.
b For score
definitions see text.
Figure captions
Figure 1. Frequency distribution of the number of kernels per ear
obtained from the successfully selfed plants in
Exp.2; greenhouse, Hohenheim 2007
Figure 2. Haploid lines in the field compared with a vigorous hybrid; Exp.I, Hohenheim 2007
Figure 3. Detail of a tassel of a haploid plant with anther score 2;
greenhouse, Hohenheim 2007
Figure 4. Ears of two selfed haploid plants ( no. 1 and 2 in Table 2); Exp.II,
Hohenheim 2007

![]() Figure 1. Frequency distribution of the number
of kernels per ear obtained from the successfully selfed
plants in Exp.II, greenhouse, Hohenheim
2007
Figure 1. Frequency distribution of the number
of kernels per ear obtained from the successfully selfed
plants in Exp.II, greenhouse, Hohenheim
2007

Figure 2. Haploid lines in the field compared
with a vigorous hybrid; Exp.I, Hohenheim
2007

Figure 3. Detail of a tassel of a haploid plant with anther score 2; greenhouse,
Hohenheim 2007
21 a 11 a
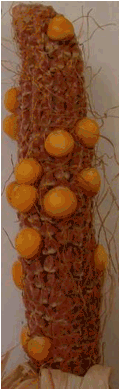

Figure 4. Ears of two selfed haploid plants (no.
1 and 2 in Table 2); Exp.II, Hohenheim
2007
Please Note: Notes submitted to the
Maize Genetics Cooperation Newsletter may be cited
only with consent of authors.