Maize Genetics Cooperation Newsletter vol 84 2010
Please Note: Notes submitted to the Maize Genetics
Cooperation Newsletter may be cited only with consent of authors.
COLUMBIA, Missouri
University
of Missouri
Chromosome Breaking Ds Sites in Maize, Revisited. Part I,
Background, Methods, Description
--Neuffer, MG
This past year I received some supplemental funding
(via NSF grant 0743804, with many thanks to Carolyn Lawrence and Mary Schaeffer
for their help in this process) to update and revise information on my maize
mutant collection that I had submitted to the Stock Center and to MaizeGDB back
in 1995. A second objective was to
provide information and stocks on a new dominant mutant collection I have
generated in the past few years, with the generous support of Sarah Hake. In the process of organizing the data I
decided it would be a good time to revisit a collection of Ds marker stocks that I had generated. The purpose of this Newsletter article is to combine
published information (Neuffer 1994, 1995) about the Ds markers with the images and case descriptions already posted at
MaizeGDB (http://www.maizegdb.org/), and
to provide a general review of the uses and expression of these stocks. This text portion will be supplemented
by information in Part II, where we use photographs of the stocks to illustrate
our discussions of the proven cases.
Part II is planned to be posted at Maize Gene Review (http://maizegenereview.org/) concurrently
with this article, and the original data at MaizeGDB will be updated at the
same time. I wish to thank Lou
Butler for assistance in gathering the data, editing the text, preparing the
artwork, and formatting this material for posting online. Thanks also are extended to Kelly Dawe
and Hugo Dooner for reading the material and providing many helpful
suggestions.
Background
of Marker Stock Development:
Loss of the normal allele in a heterozygote to
produce a hemizygote with the recessive phenotype can be very useful in genetic
studies. This may be produced in a
number of ways, including x-radiation, marked ring chromosomes, and B-A
chromosome translocations. The
chromosome-breaking Ds elements are
especially useful in studying the expression of lethal mutant tissue in
chimeras produced by loss of the normal allele in a heterozygote. Our purpose in this project was to
generate chromosome breakage in heterozygous, lethal embryo, defective kernel (dek) whole kernel maize mutants on all
chromosome arms. Plant chimeras of
hemizygous mutant whole kernel and seedling -/dek
tissue, sustained by adjoining normal +/dek
tissue, could thus be observed and compared. By taking advantage of its chromosome breaking properties,
we were able to produce Ds markers on
many genetically marked chromosome arms (Table 1).
The transposable element Ds, discovered and analyzed in detail by McClintock (1951), is the
responding element of the Ac Ds system. It has the unique property of being able to move about
(transpose) in the genome when Ac is
present. Thus, sites throughout the genome can be selected for further genetic
analysis. At the resident site Ds can also suppress the function of an
associated active gene and/or cause a break in the chromosome at that site,
initiating the breakage-fusion bridge-cycle described by McClintock (1941; for
diagram see http://profiles.nlm.nih.gov/LL/B/B/R/S/) of sequential chromosome breaks
with associated losses and consequent gain of genetic material in the daughter
cells of a mitotic or meiotic division.
Mutations induced by Ds at the
gene site are observable when they interfere with the gene�s function,
producing a recessive null phenotype.
This loss of genetic function in a heterozygote with Ds on the homolog with the dominant
allele allows a recessive allele to be uncovered as a chimera of recessive
tissue. In some cases Ds acts as both
breaking and suppressing. If the
breakage feature is associated with suppression of the resident gene, this can
affect observation of the genes used to mark chromosome loss.
It is complicated to interpret results from Ds experiments. The state of activity, variations in Ac dosage, and other genetic modifiers
can all lead to variation at the insertion or neighboring site. Moreover, the characteristics of each
variation depend on the marker used, the relative position of Ds and the marker on the chromosome arm,
and on other types of genetic modifiers. Originally we intended to find a site
between the marker and the centromere, so that the marker would be lost as an
early Ds event. It became clear, however, that the
size, frequency, and characteristics of sectors shown at each new Ds site were related to the position on
the chromosome arm relative to the marker used. In fact, expression was related to the position of three
components: Ds, the marker, and the centromere. This position could be determined by the type of loss
pattern in the kernel. Location on
the distal side of the arm from the centromere was observable as frequent,
small sectors, associated with twin duplicate-deficiency spots. Proximal location was characterized by
frequent large sectors along with normal tissue in single dots, or dots in
chains or clusters within the large deficient sector. Location at the gene site was usually accompanied by
suppression of the gene function to produce the equivalent of the recessive or
null allele that was exceptionally unstable when Ac was present, producing frequent reversions to some level of
mutant gene expression. These
appeared as sectors, usually dotted, of normal tissue on the null kernel
background or elongated dominant streaks of red on the pericarp and anthocyanin
on normal green plant leaves.
Methods and
stock preparation:
The
original Ds1 site described by
McClintock (1951) was located proximal to Wx1
on the short arm of chromosome 9.
My stock of this original Ds1
material has been termed Ds-9S1. Dr. Jerry Kermicle generously provided
two additional stocks that were presumed to carry the Ds1 site on the long arm of chromosome 10 near the g1 locus; these were used to generate
the remaining Ds stocks. One stock (P1-vv, Ds-10L2 R1-sc with Ds 10 cM proximal to g1) carried Ds on the long arm of chromosome 10, proximal to the centromere
from the R1-sc allele at the R1 locus and the other stock was P1-vv, Ds-10L4 R1-sc with Ds in the same general region. The
pollen source was P1-vv/P1-wr, Ds-10L2 R1-sc/Ds-10L2 R1-sc or R1-r and
homozygous dominant for all the markers in the tester stocks used except
chromosome 2S (see below).
Heterozygous P1-vv was used in
order to retain the early large sector properties of 1 dose of Ac, thus increasing the likelihood of
gametic events. The probability of
capturing duplicate events from a single large tassel sector was minimal since
cases were used from trials in two or more seasons and by selecting for
visually different characteristics.
All the genes used as marker stocks were present as dominant alleles in
the pollen stock, except for chromosome 2S, where a special pollen stock was
prepared using the dominant aleurone color allele B1:Peru. Since B1:Peru and R1-sc are duplicate factors, we used a b1 r1 tester. Changes
of aleurone color B function would
appear as a color change on ears with purple kernels.
The Ac
used was the one associated with P1-vv
and in many of the Ds cases P1-vv can be clearly seen as red streaks
on the colorless kernels. This is
an expression of P1-vv in the
maternal parent and is caused when Ac
is inserted in or near the red pericarp locus. The streaks are caused whenever Ac moves away from the locus. Ac has most of the same properties as Ds and can act an autonomous
element.
Vigorous testers homozygous for the appropriate recessive
aleurone, endosperm, and seedling markers were prepared. If the tester mutant was lethal (dek1, w3, o5) normal kernels
from a segregating F2 were used.
Of these, 2/3 would be heterozygous (1/2 correct gametes), and 1/3 would
be discarded as homozygous normal.
For each tester, 100 or more plants were used as female parent. These
were grown in an isolated, open-pollinated, detasseled plot according to the
method of Stadler (1946). The ears
were marked for each of the 16 chromsome arms that carried a usable
marker. They were examined for two
types of single kernel events: (1) Transposition of Ds near the marker and the centromere. Depending on the particular
tester stock, mosaicism or sectoring for purple vs. colorless or bronze
aleurone, and normal vs. shrunken, brittle or collapsed endosperm would reflect
chromosome breaking activity in the endosperm, and many would be expected to
have the same activity in the embryo thereby transmitting it to the next
generation. (2) Transposition of Ds
to the marker site which would suppress the dominant allele. This would result in a recessive mutant
case with dominant revertant sectors due to the presence of Ac. Those without dots and revertant sectors are due to the
absence of Ac because of chance
segregation in gamete formation.
They were considered potentially valid recessive mutable cases that
could potentially show their mutability when recombined with Ac.
Single kernel cases were planted and observed for
any variations from normal plant phenotype. Any kind of sectoring was especially noted as potentially
indicating Ds activity in the
plant. Plants were selfed and
backcrossed to their respective tester for confirmation of Ds activity. Stable
recessive cases were crossed to an Ac
stock to test revertability.
Seedling
markers were used in cases where endosperm markers were not available for that
chromosome. All kernels were
planted in sand benches and examined for seedlings of two kinds: 1) whole seedling mutant cases with
multiple recessive sectors (Ac) and
without sectors (no Ac); and 2)
normal green seedlings with chimeras of recessive tissue for the marker
used. Observed cases were
transplanted to pots and were grown to maturity. The mutant seedlings for lethal phenotypes died, except when
there was adequate revertant tissue to support plant growth. Those seedlings that survived to
maturity were selfed and backcrossed to the recessive tester to confirm.
Many
putative cases were observed in most of the tester stocks included in the
crosses. Any that survived were
grown to maturity and tested. The
precision of observations varied greatly due to variation in the mutant. The aleurone color stocks gave excellent
mosaic kernels with colorless or bronze sectors on part of the endosperm or
aleurone layer (which is a part of the endosperm), and those with shrunken or
brittle endosperm were also fairly good.
However, the collapsed and opaque endosperm cases were difficult to
recognize and many ambiguous cases were tested, most of which failed confirmation. Losses of other kernel cases to normal
field conditions forced us to abandon any efforts at quantitative measurement
of the frequency of events involving markers on individual arms.
The
Non-transmitting cases:
There were 79 kernel cases which failed to
transmit; these were thought to be misinterpretations or nontransmission. However, recent reconsideration of
these cases led to another interpretation. The non-transmitting cases should have been included,
because we now know that losses occur as a consequence of nuclear division
separating unequal products. We
originally looked only for those cases where losses were apparent or where the
product was associated with a mutant phenotype. The other product of the event would be a duplication or
some other variation that often did not have an immediate phenotype. These cases should have been examined
for unusual phenomenon and/or delayed expression. Photographs were taken of all
of these cases and data from these apparent non-corresponding cases will be
revisited to consider what evidence we still have and what that evidence points
to. This information will be
presented in a later publication.
Observations:
Usually Ds
showed a chromosome breaking property, but cases of suppression, either with or
without breakage, were also observed.
Ds losses appeared to occur
much sooner in the kernel (larger sectors) than in the seedling (smaller
sectors). This observation,
however, could merely reflect the observed relative maturity of the respective
tissues. Determining whether a
mutable allele due to suppression was also a chromosome breaker related largely
to the properties of the marker gene.
For example, identifying twin spots required different dosage levels of
gene expression. As we accumulated
chromosome breaking Ds cases for arms
using aleurone color markers we observed two major types of aleurone color
mosaics: large mutant sectors
indicated early breakage events, while smaller sectors indicated later
events. These were distinct from
timing changes in Ds events resulting
from changes in Ac dosage, because
our material usually had only one dose of Ac
from the male parent and therefore should have had only large sectors. This was not always true, as other
factors such as genetic modifiers and an unidentified independently segregating
Ac, which occasionally appeared in
the stocks used.
Several types of Ds cases were observed.
There was a high frequency of large sectors. According to a personal communication from Dr. Jerry
Kermicle, the original Ds-10L2 R1-sc case similarly displayed a high
number of large sectors. The
kernels appear more colorless than colored due to the large amount of tissue with
lost gene function. The Ds site was
shown to be proximal (between the marker and the centromere). We also observed an unusual mosaic
pattern, which we originally thought was cases of parental colored tissue with
repeated subsequent loss of color.
However, these were actually found to be chains, clusters and/or islands
of colored tissue (Figures 1, 2) which we interpreted to be retention of the
functional gene in acentric fragments. These fragments were carried along in
daughter cells as clones from the initial break. The clusters, chains and islands were as a rule more
intensely pigmented for those markers having a dilution dosage effect (i.e., C2, R1-scm,
B1:Peru), to be expected because the
acentric fragment from a proximal break would carry duplications for the gene
being followed. These observed
islands of normal tissue within a sector of mutant tissue were similar to those
seen many years ago by L.J. Stadler and also by me, his student. We were
performing experiments involving radiation-induced color losses and were unable
to interpret the meaning of the spots.
Stadler called these islands "recovery spots� (Figure 2). We
observed that the frequency and size of these recovery spots depended on the
chromosome arm and the proximity of the marker, and therefore the Ds site to the centromere. It appears that short acentric
fragments are not retained or �recovered� as frequently as long ones.
Other Ds
cases displayed a relatively high frequency of medium and small sectors, and a
low frequency of large sectors.
For genes with a dosage effect on aleurone expression (for example, Ds-4L6 with a C2 marker, and Ds-1S3),
the mosaic kernels were dilute (depending on the dosage threshold for gene
expression of the gene studied) with a few large sectors and many small
colorless sectors or patches scattered randomly over the aleurone layer. Considering that only one Ac was present, these were rather late
events. The small patches ranged
from about 1/32 of the kernel surface to those composed of only a few aleurone
cells. Large sectors could cover
from 1/4 to 1/3 of the aleurone surface, but these were rare.
Frequently, the colorless patches were angular and
sharply outlined, and were edged by a smaller and intensely pigmented
sector. The smaller sector was
clearly on the dilute side of the border rather than on the colorless
side. These spots of colorless and
intense pigmentation arising from a dilute background were termed twin spots
(Figure 3). McClintock (1941)
explained these spots to represent a deficiency and duplication for the marker
gene which occurred as a result from a break in the chromosome distal to the
marker. The broken ends then
rejoined, forming a bridge at mitotic anaphase with two copies of this
marker. Then, a second non-median
break occurred at telophase such that both copies of the gene went to one
daughter cell and none to the other.
References:
MCCLINTOCK, B. 1941. Spontaneous alterations in
chromosome size and form in Zea mays.
Cold Spring Harbor Symp. Quant. Biol. 9:72-81.
MCCLINTOCK, B. 1951. Chromosome organization and
genic expression. Cold Spring Harbor Symp. Quant. Biol. 16:13-47.
NEUFFER, M.G. 1994. Chimeras for genetic analysis,
pp. 258-262. In: The Maize Handbook, M. Freeling and V. Walbot, eds.,
Springer-Verlag, New York.
NEUFFER, M.G. 1995. Chromosome breaking sites for
genetic analysis in maize. Maydica 40:99-116.
STADLER, L.J. 1946. Spontaneous mutation at the R locus in maize. I. The aleurone-color
and plant-color effects. Genetics 31:377-394.
Table 1:Transposition
sites for chromosome breaking Ds
stocks.
Symbol Marker Position
Ds-1S1 Dek1 distal
Ds-1S2 Dek1 probably
distal
Ds-1S3 Dek1 distal
Ds-1S4 Dek1 proximal
Ds-1L1 Bz2 proximal
Ds-1L2 Bz2 at
the Bz2 (bz2-m3) locus
Ds-1L3 Bz2 at
the Bz2 (bz2-m3) locus
Ds-1L6 Bz2 at
the Bz2 (bz2-m) locus
Ds-2S1 B1:Peru distal
Ds-2S2 B1:Peru unknown
Ds-2S3 B1:Peru at
the B1:Peru (b1-m1) locus
Ds-2S4 B1:Peru at
the B1:Peru (b1-md2) locus
Ds-2L1 W3 unknown
Ds-3L1 A1 Sh2 proximal
Ds-3L2 A1 Sh2 proximal
Ds-4S1 Bt2 unknown
Ds-4S2 Bt2 unknown
Ds-4L1 C2 distal
Ds-4L3 C2 at
the C2 locus
Ds-4L4 C2 distal
Ds-4L5 C2 distal
Ds-4L6 C2 distal
Ds-4L7 C2 distal
Ds-5S1 A2 proximal
Ds-5S2 A2 proximal
Ds-5L1 Bt1 distal to Bt1 and proximal to Pr1
Ds-7L1 O5 distal
Ds-7L2 O5 proximal?
Ds-7L3 O5 proximal
Ds-8L1 Pro1 unknown
Ds-9S1 C1-I proximal?
Ds-9L2 Dek13 unknown
Ds-10L2 R1-sc proximal
Ds-10L4 R1-sc proximal
Ds-10L5 R1-sc Ac at the R1 locus
Ds-10L6 R1-sc Ac at the R1 locus
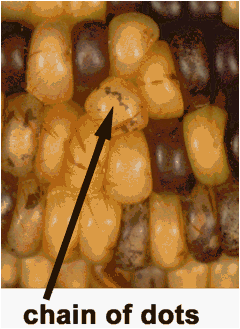
Figure 1: A good example of chains of dots.
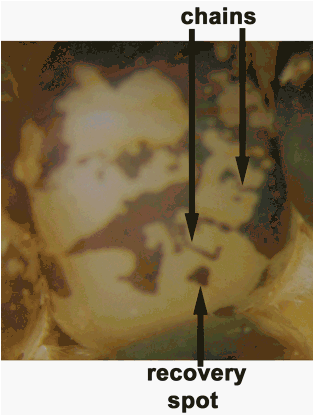
Figure 2: Two examples of chains and an example
of a recovery spot.
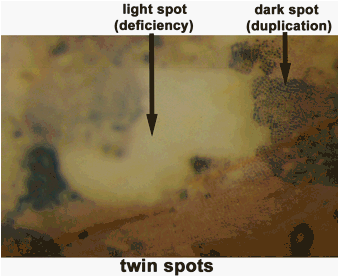
Figure 3, typical expression of twin spots.