Maize Genetics
Cooperation Newsletter vol 84 2010
Please Note: Notes
submitted to the Maize Genetics Cooperation Newsletter may be cited only with
consent of authors.
LLAVALLOL, ARGENTINA
Instituto Fitot�cnico de Santa Catalina
Facultad de Ciencias Agrarias y Forestales. Universidad Nacional de La
Plata. CC4 (1836) LLavallol.
The use of electrolyte leakage in the
evaluation of salinity tolerance at seedling stage in maize (Zea mays L.).
-Collado, MB; Aulicino, MB; Molina, MC;
Arturi, MJ.
Maize is classified as a salt-sensitive crop
plant (Maas & Hoffman 1977). The response of maize to salinity varies depending
on the stage of development (Kaddah & Ghowail 1964; Maas et al. 1983; Pasternak, Malach & Botovic 1985).
Vegetative growth appears to be most sensitive to salinity, while plants are much
less affected at later stages (Cramer 1994). The development of salt tolerance crop plant
cultivars has been proposed as the most effective strategy to overcome this
problem (Epstein & Rains, 1987). The resistance to abiotic stress in
general and to salinity stress in particular is under polygenic control
(Flowers & Yeo, 1995).
Munns (1993) has proposed a biphasic model of
growth response to salinity. The growth reduction in the first phase is an effect
of salt outside rather than inside the plant (osmotic phase). In the second
phase, the concentration of toxic ions increases
rapidly, especially in old leaves, which die as a result of a fast increase of
the salt concentrations in the cell wall or cytoplasm when vacuoles can no
longer sequester incoming salts (ionic phase). In this second phase, genotypes
which vary in salt tolerance may respond differently as a result of their different
abilities to exclude toxic ions or to sequester them in the vacuoles (Munns
1993).
The tolerance to salinity could be classified
in three mechanisms:
1.
Tolerance to
osmotic stress: the mechanisms controlling this phase are not specific to
salinity; they are associated with water stress.
2.
Na+
exclusion from leaf blades: the Na+ is accumulating by the root and
this protected the leaves to arise the salt to toxic level.
3.
Tissue tolerance:
the tissue tolerance to accumulated Na+, the ion is
compartmentalization at cellular and intracellular level to avoid toxic
concentration within the cytoplasm (Munns & Tester, 2008).
Different typical agronomic selection
parameters for salinity tolerance are being used: yield, survival, plant height
(Noble and Rogers, 1992), leaf area (Franco et al., 1993), injury (Munns,
1993), relative growth rate in stress studies in different crops (He and Cramer,
1992). However, it is not yet possible to find any sensitive criterion that
could reliably be used by breeders to improve salt tolerance of plants (Ashraf
& Harris, 2004). Recently, several traits like: shoot K concentration (Bagci
et al., 2007),
photosynthetic capacity (Ashraf et al., 2007) and cell membrane stability (Aslam et al., 2006) in maize have been considered as a
reliable parameter for salt tolerance studies.
Salt tolerance, studied by measuring
cell membrane stability, has shown changes in the structure or composition of
the membrane in genotypes with different response in salinity conditions. Salt
sensitive cultivars show greater increase in the cell permeability compared to
salt tolerant cultivars. This trait could be reflected in the behaviour of the
whole plant and could be a useful feature in a breeding program for developing
salt tolerance genotypes (Mansour and Salama, 2004; Mansour et. al., 2005).
This paper examines the use of the electrolyte leakage (Cell membrane stability)
trait in the
selection at seedling stage that may be important in the screen for different
mechanisms of tolerance in plants exposed to salinity.
Eight accessions/lines were used
five of which were populations and three inbred lines. Seeds of the different
genotypes were surface sterilized in 1% sodium hypochlorite solution for 5
minutes before experimentation, then rinsed with distilled water. Three seeds
were planted in each pot containing perlit; these pots were put in trays with a
nutrient solution. Two treatments were applied: control (cont.) where no ClNa solution
was added and the other treatment receiving 100mM ClNa (salt). The experimental
was carried out in controlled environmental room at 25 �C, with 16 day length
and with a relative humidity of 60%.
After 14 days of each salt
treatment, the seedlings were harvested. The length for shoot and radicle (SL
and RL, respectively) were recorded. Shoot and radicle were separated and the
samples were dried for two days until constant weight, for dry mass
determination (DS and DR respectively).
The cell membrane stability was
estimated on the third leaf. A piece of leaf was cut, weighted and washed with
distilled water to remove the solution from tissue, then the samples were immersed
in 10ml of distilled water and placed for incubation at 10�C for 24hs. After
incubation samples were equilibrated to room temperature. Then, the electrical conductivity of the medium was recorded (EC1), with
a portable EC meter (Consort C931). The samples were autoclaved for 15 min to
kill all tissues, and after cooled to room temperature, the conductivity of the
solutions was read again (EC2). Electrolyte
leakage (%) was calculated as: EL = (EC1/ EC2) × 100. The electrolyte leakage was
measured.
The data were subjected to an
analysis of variance and the means were compared by the least significance
differences test (LSD) at a 5% level (Sokal and Rolf, 1995).
The ANOVA pointed out that although
the tested genotypes have shown significant and highly significant differences
among themselves, in the salinity treatment, the comparison of both treatments
has resulted non-significant for most of the traits that were tested, with
exception of RL, EC1 and EL (Table 1).
Consequently, these traits would be extremely
useful in salinity tolerance improvement programs, especially Root Length which
has shown a major growth reduction compared to the controls. This apparently
evidences the importance of the Root Length variable in the identification of a
tolerant response, as pointed out by various authors (Rao and McNelly, 1999; Khan and McNelly, 2003).
Las mediciones de da�o de membrana estar�an asociadas con la
susceptibilidad a la sal a nivel celular. El gr�fico N�1 muestra que el
genotipo F564 podr�a ser considerado
como susceptible, por que fue el
que mayor valor de EC1 present� y en consecuencia mayor da�o por salinidad. En
cambio, SC75 present� el valor m�s bajo de EC1 por consiguiente, tendr�a una
menor p�rdida de electrolitos lo que indicar�a un comportamiento tolerante. Sin
embargo, cuando se analizan los par�metros de crecimiento, en especial el RL
puede observarse que estos dos genotipos fueron los que menos p�rdida de
crecimiento sufrieron. En consecuencia, ambos genotipos ser�a tolerantes a
salinidad pero asociada probablemente a mecanismos diferentes de tolerancia. La
l�nea SC75 no habr�a sufrido gran
da�o en membrana debido probablemente a que no habr�a acumulado en forma excesiva Sodio en la parte �rea, durante
el tiempo de exposici�n a sal este i�n se acumul� en ra�z (mecanismo de
exclusi�n de sodio). En cambio, F564 sufri� un importante da�o en membrana, que
podr�a asociarse a una acumulaci�n de sodio en parte a�rea (vacuola) sin
afectar grandemente el metabolismo celular (tolerancia de tejidos al sodio).
Measurement of
membrane damage seems to be associated with salt sensitivity at cellular
level. Figure 1 shows that genotype F564 could be considered sensitive
because it showed the highest level of EC1 and, consequently, the most
damage due to salinity. On the other hand, SC75 showed the lowest level of EC1
and therefore, it has a lower electrolyte leakage which indicates a
tolerant behavior. However, when growth parameters are analyzed, in
particular RL, it can be observed that these two genotypes were the ones that
suffered the least growth loss. As a result, both genotypes appear to be
tolerant to salinity but probably associated with different tolerance
mechanisms. The SC75 line did not suffer great membrane damage, since it
probably did not accumulate an excessive amount of Na+ in the shoot during the period
of exposure to salt, this ion was accumulated in the root (sodium exclusion
mechanism). Instead, F564 suffered significant damage in the membrane, which
could be associated with an accumulation of sodium in shoot (vacuole) without
severely affecting the cellular metabolism (tissue tolerance to sodium).
Ashraf, F.M. & Mc Neally, T.
1987. Variability for salt tolerance in Sorghum bicolor (L.) Moench. Under
hidroponic conditions. J. Agron. & Crop Sci. 159:269-277.
Ashraf, M. & Mc Neally, T. 1990.
Improvement of salt tolerance in maize for selection and breeding. Plant
Breed., 104(2):101-107.
Ashraf, M. and P.J.C. Harris. 2004. Potential
biochemical indicators of salinity tolerance in plants. Plant Sci., 166: 3-16.
Ashraf, M., S. Nawazish and H.R. Athar. 2007.
Are chlorophyll fluorescence and photosynthetic capacity potential
physiological determinants of drought tolerance in maize (Zea mays L.). Pak. J. Bot., 39(4): 1123-1131.
Ashraf, M. 2009. Biotechnological approach of
improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv.,
27: 84-93.
Aslam, M., I.A. Khan, M. Saleem and Z. Ali.
2006. Assessment of water stress tolerance in different maize accessions at
germination and early growth stage. Pak. J. Bot., 38(5): 1571-1579.
Bagci, S.A., H. Ekiz and A. Yilmaz. 2007. Salt
tolerance of sixteen wheat genotypes during seedling growth. Turk. J. Agric.
For., 31: 363-372.
Cramer G.R. 1994. Response of maize (Zea
mays L.) to salinity. In Handbook
of Plant and Crop Stress (ed. M. Pessakli). pp. 449-59, Marcel Dekker. New
York.
Epstein, E. & Rains, D. 1987. Advances in salt tolerance. Plant
& Soil, 99:17-29.
Flowers, T.J. & Flowers, S.A. 2005. Why does salinity pose such a difficult problem for
plant breeders? Agric. Water Maneg., 78:15-24.
Flowers, T.J. & Yeo, A.R. 1995. Breeding for salinity
resistance in crop plants: where next? Australian Journal of Plant Physiology, 22:875-884.
Kaddah M.T, & Ghowail S. I. 1964. Salinity
effects on the growth of corn at different stages of development. Agronomy
Journal, 56: 214-217.
Maas E,V, & Hoffman G. J. 1977. Crop salt tolerance-current assessment. Journal
of the Irrigation and Drainage Division ASCE, 103 (IR2). I 15-1.34,
Maas E,V, & Hoffman G.J. 1983. Salt sensitivity of corn at various growth
stages, California Agriculture,
37: 14-15.
Maas E.V., Hoffman GJ, Chaba G.D., Poss J.A.
& Shanon M.C. 1983. Salt sensitivity of corn at various growth stages. Irrigation
Science, 4: 45-57,
Mansour M.M., K.H. Salama, 2004
Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 52: 113-122.
Mansour M.M., K.H. Salama, F.Z.
Ali, A.F. Abou Hadid, 2005 Cell and plant response to Na Cl
in Zea mays L.
cultivars differing in salt tolerance. Gen. Appl. Plant Physiology 31: 29-41.
Munns R. 1993. Physiological processes limiting
plant growth in saline soils: some dogmas and hypotheses. Plant, Cell and
Environment, 16:15-24.
Munns R.,. Tester M, 2008 Mechanisms
of salinity tolerance. Annu. Rev. Plant Biol. 59: 651-681.
Schubert S, & Lauchli A. 1986. Na+exclusion.
H+ release, and growth of two different maize cultivars under NaCl
salinity. J. Plant Phisiol. 126:145-154.
Pasternac D, De Malach Y,
& Borovic L. 1985. Irrigation
with brackish water under de.seit conditions, II Physiological and yield
response of maize {Zea mays) to
continuous irrigation with brackish water to altering brackish-fresh-brackish
water irrigation. Agricultural Water Management, 10: 47-60.
Paterniani, E. 1990. Maize breeding
in tropic. Cri. Rev. Plant Sci. 9:125-154.
Rao S.A., T. Mcneilly, 1999 Genetic
basis of variation for salt tolerance in maize (Zea mays L). Euphytica 108: 145- 450.
Khan A.A., T. Mcneilly, 2005 Triple
test cross analysis for salinity tolerance based upon seedling root length in
maize (Zea mays L.).
Breeding Science 55: 321-325.
Sokal R.R., F.J. Rolf, 1995
Biometry, Third ed. W.H. Freeman and Co., New York.
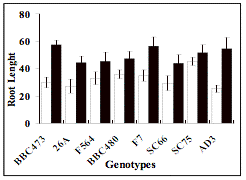
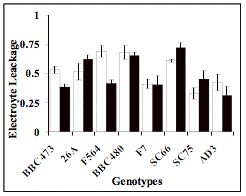
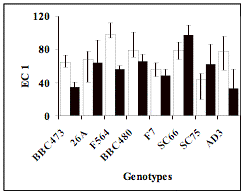
Figure 1:
Average of each genotypes for control (black bars) and salt (white) treatments
of the traits: Lenght of Root (RL), Electrolyte
Leakage (EL) and Electrical Conductivity 1 (EC1). Vertical bars are the S.D. of
four replications.