Maize Genetics Cooperation Newsletter vol 81 2007
IRKUTSK, RUSSIA
Institute of Plant
Physiology and Biochemistry
Presumable redox control of
phosphorylation of the mitochondrial chaperonin hsp60
--Subota, IY; Arziev, AS; Sengenko,
LP; Tarasenko, VI;
Konstantinov, YM
It was shown previously (MNL 80:14-15) that
phosphorylation/dephosphorylation of serine/threonine or histidine residues of
the target mitochondrial proteins is presumably involved in the metabolic
response of mitochondria under the changes of redox conditions. To date redox-dependent phosphorylation
of mitochondrial proteins has not been sufficiently elucidated. Although this modification has been
observed in our experiments for at least 8 maize mitochondrial proteins (MNL
80:14-15), the nature of the polypeptides and the function of phosphorylation
for these proteins remain poorly understood. In this work, we show that one of the mitochondrial phosphoproteins is the heat shock protein
60 (hsp60).
The mitochondria were isolated
from 3-day-old etiolated maize seedlings of hybrid VIR42MV, by a standard
method of differential centrifugation.
Protein phosphorylation assays were carried out according to Struglics
et al. (FEBS Lett. 475:213-217, 2000) with the use of [γ32P]ATP at 6000 Ci/mmol.
By immunoblotting with specific
antibodies, we have identified one of 8
mitochondrial phosphoproteins as mitochondrial
chaper-
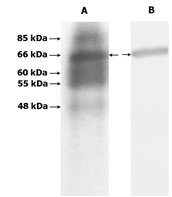
Figure
1. In vitro phosphorylation of
redox-sensitive phosphoproteins including 66 kDa (A) were resolved by 12% SDS/PAGE and were immunoblotted (B) with antibody against hsp60.
onin hsp60 (Fig. 1).
Mitochondrial chaperonin hsp60 is required for ATP-dependent folding of
precursor polypeptides and complex assembly. It also prevents aggregation and mediates protein refolding
after heat shock. There is also
some evidence of hsp60 involvement in the structure and transmission of
mitochondrial DNA nucleoids in Saccharomyces
cerevisiae (Kaufman et al., J. Cell. Biol. 163:457-461, 2003). We suggest that this evolutionarily
conserved hsp60 participates in redox regulation of mitochondrial genome expression
and is possibly mediated by reversible redox-dependent phosphorylation.
Please Note: Notes
submitted to the Maize Genetics Cooperation Newsletter may be cited only with
consent of authors.